Patent & IP news for September 28, 2017
Patent Litigations
- No new litigations this day!
USPTO Weekly Stats
7,383
published
appl'ns
appl'ns
5,798
granted
patents
patents
64
ptab
decisions
decisions
Patent & IP Blogs
 Proposed EMA relocation: staff survey update from ipkitten.blogspot.com
Proposed EMA relocation: staff survey update from ipkitten.blogspot.com Readers in the life science sector will be aware of the proposed relocation of the EMA away from London to another European city in the wake of the Brexit referendum vote. This week, the EMA ...
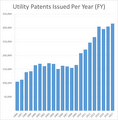 FY2017: Record Year for PTO Grant Numbers from patentlyo.com
FY2017: Record Year for PTO Grant Numbers from patentlyo.com The USPTO has completed its patent issuances for Fiscal Year 2017 which ends for the Federal Government on September 30, 2017. The primary raw number 315,386 utility patents issued in FY 2017. This is ...
 Medical data in a twist - Technomed v Bluecrest from ipkitten.blogspot.com
Medical data in a twist - Technomed v Bluecrest from ipkitten.blogspot.com Other types of base are availableData was recently described by The Economist as as the new oil. Every valuable asset tends to be protected by IP in some shape or form and data* is no ...
Four Stages To Monetizing A Patent Portfolio from www.ip-watch.org
By Martin Bijman, Director, Intellectual Property Products , TechInsights Successfully pursuing the monetization of IP assets requires an accurate assessment of their value and position within the marketplace. Essentially, monetizing a patent portfolio includes four key ...
By Martin Bijman, Director, Intellectual Property Products , TechInsights Successfully pursuing the monetization of IP assets requires an accurate assessment of their value and position within the marketplace. Essentially, monetizing a patent portfolio includes four key ...
Disparity In Access To Medicines Spurs “Humanitarian” Patent Licensing from www.ip-watch.org
“There are shameful access disparities around the world” to life-saving medicines, Harvard University Global Access in Action project Co-Director Quentin Palfrey said at a 26 September Center for Strategic and International Studies event in Washington ...
“There are shameful access disparities around the world” to life-saving medicines, Harvard University Global Access in Action project Co-Director Quentin Palfrey said at a 26 September Center for Strategic and International Studies event in Washington ...
How USPTO Patent Reviews Became Imperiled from www.ip-watch.org
Initially, the lawsuit was widely viewed as a waste of time. The suit asserted a strained legal argument that already had been rejected twice by federal appellate panels, in 1985 and 1992. Yet this lawsuit ...
Initially, the lawsuit was widely viewed as a waste of time. The suit asserted a strained legal argument that already had been rejected twice by federal appellate panels, in 1985 and 1992. Yet this lawsuit ...
Defendant’s Decision to Pursue IPR Bars Pursuit of § 285 Attorney Fees Award from docketreport.blogspot.com
Following inter partes review, the court granted plaintiff's motion to dismiss its patent infringement action and defendant's invalidity and noninfringement counterclaims as moot and rejected defendant's request to allow 90 days of ...
Following inter partes review, the court granted plaintiff's motion to dismiss its patent infringement action and defendant's invalidity and noninfringement counterclaims as moot and rejected defendant's request to allow 90 days of ...
Waiting for Outcome of Rule 12 Motion Waives Objection to Improper Venue from docketreport.blogspot.com
The court denied defendant's motion to dismiss or transfer plaintiff's patent infringement action for improper venue because defendant waived its venue defense by waiting for the outcome of its earlier Rule 12 motion ...
The court denied defendant's motion to dismiss or transfer plaintiff's patent infringement action for improper venue because defendant waived its venue defense by waiting for the outcome of its earlier Rule 12 motion ...
New EU Commission Guidelines On Illegal Content Online Clarify Liability For Online Platforms from www.ip-watch.org
The European Commission today issued guidelines for removing illegal content online, largely following the lines of existing rules and guidance, but hinting at a possible future move to harmonise practices in this area. Technology companies ...
The European Commission today issued guidelines for removing illegal content online, largely following the lines of existing rules and guidance, but hinting at a possible future move to harmonise practices in this area. Technology companies ...
More Oil States History: First US Patent Case was a Revocation Proceeding from patentlyo.com
Prof. Christopher Beauchamp has added further to the historical analysis relevant to the question of whether it the AIA-trials – trial-like administrative patent revocations – are Constitutionally proper. That question juxtaposes contemporary expansive administrative law against the ...
Prof. Christopher Beauchamp has added further to the historical analysis relevant to the question of whether it the AIA-trials – trial-like administrative patent revocations – are Constitutionally proper. That question juxtaposes contemporary expansive administrative law against the ...
HUMIRA® Biosimilar Update -- Settlement in AbbVie v. Amgen Case Announced and AbbVie v. Boehringer Ingelheim Litigation Begins from www.patentdocs.org
By Andrew Williams -- Earlier today, both parties to the AbbVie v. Amgen litigation announced a settlement that resolves all intellectual property-related litigation over Amgen's FDA-approved adalimumab biosimilar AMGEVITA™/AMJEVITA™ (see AbbVie press release & Amgen ...
By Andrew Williams -- Earlier today, both parties to the AbbVie v. Amgen litigation announced a settlement that resolves all intellectual property-related litigation over Amgen's FDA-approved adalimumab biosimilar AMGEVITA™/AMJEVITA™ (see AbbVie press release & Amgen ...
Some content © 2007–2017 RPX Corporation.
Terms of Service & Privacy Policy
For DMCA requests contact help@priorsmart.com.
Terms of Service & Privacy Policy
For DMCA requests contact help@priorsmart.com.
